








Alpha Helix & Beta Sheet Structure Sets©
With our Alpha Helix – Beta Sheet Models, your students will explore how the repeating N-C-C pattern can result in two very different secondary structures! They’ll further discover how the two backbone structures provide a stable scaffold upon which tertiary structure forms with side chains.
The Secondary Structure Set is a plastic two-model set with CPK colored backbone atoms. The accompanying slide deck allows you to guide a whole-class activity or provide a differentiated opportunity for students who want more.
Your students will:
-
Compare and contrast four different protein structures – beta globin, green fluorescent, insulin, and zinc finger
-
Review four levels of protein structure
-
Examine the models, and record their observations and questions
-
Find and count the backbone of each amino acid
-
Trace the structures from their N-terminus to C-terminus and discover the parallel and antiparallel strands
-
Learn the stabilizing importance of hydrogen bonds
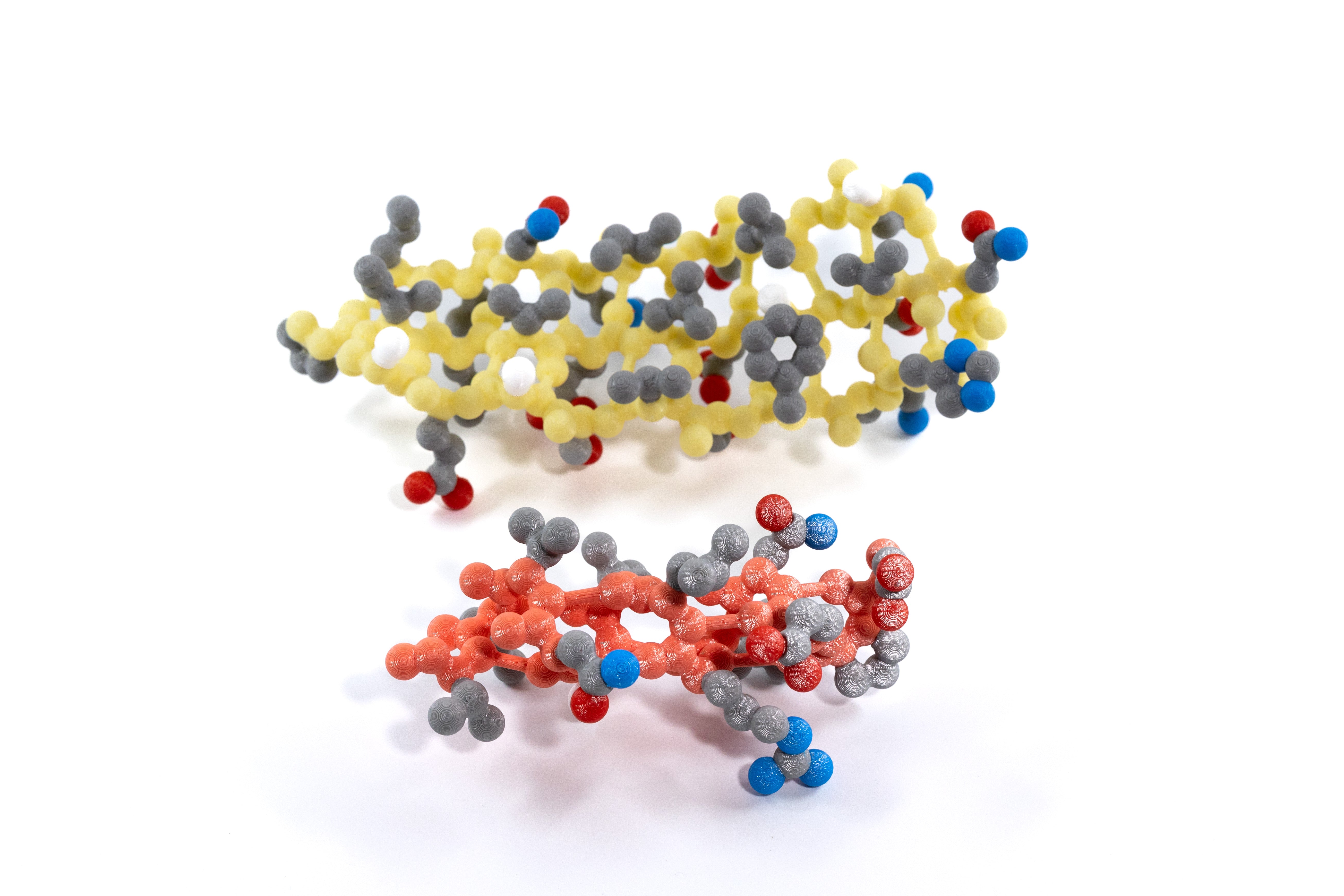
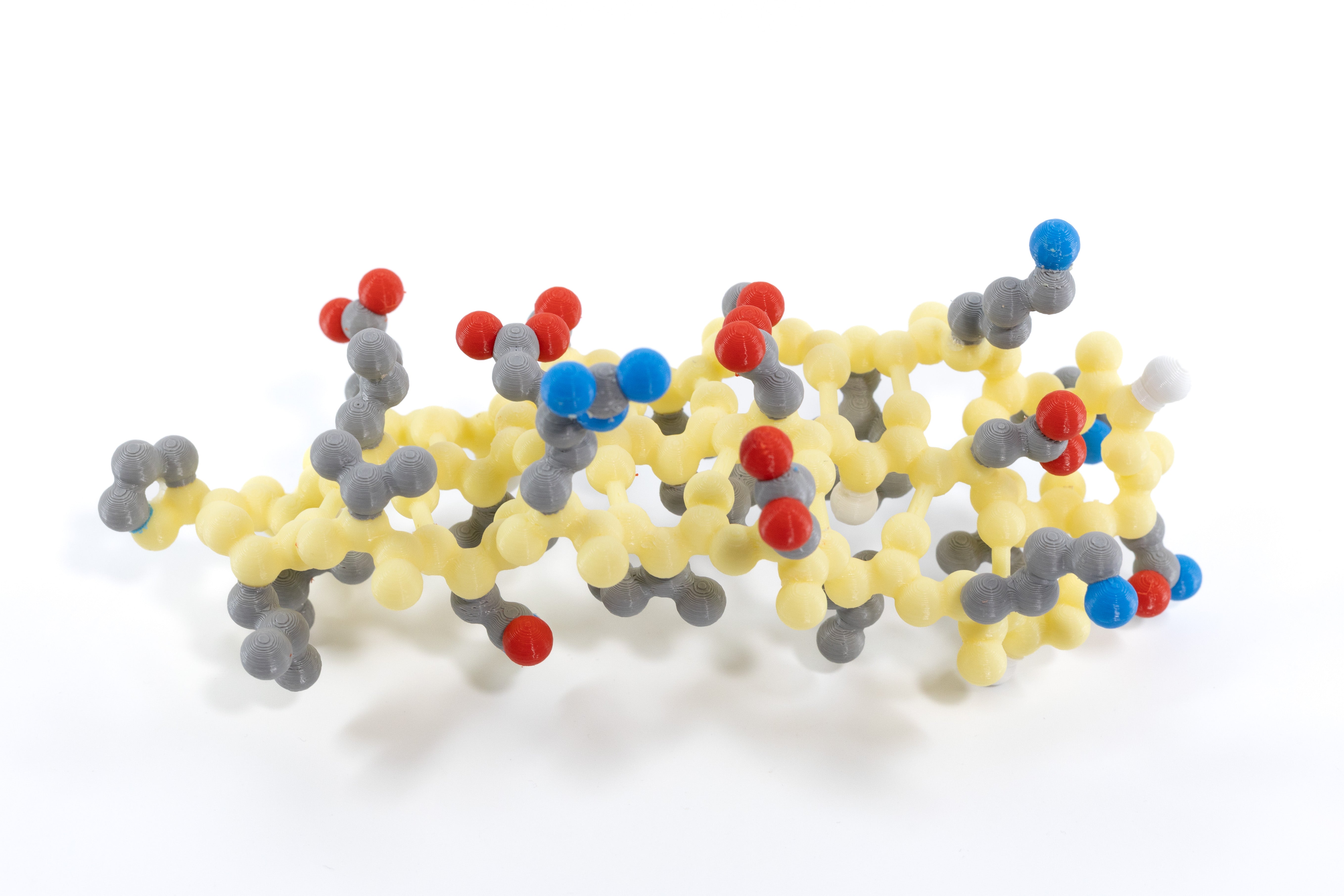
With the Tertiary Structure Set your students will search for patterns based on the hydrophobic or hydrophilic properties of each backbone’s side chains. In this set, the side chains are CPK colored, and the backbone atoms are light red in the alpha helix and yellow in the beta sheet. With these color schemes, the side chains’ atoms and resulting properties stand out, helping your students understand how the chemical properties of amino acid side chains influence protein folding and stability.
Your students will:
-
Examine the models, and record their observations and questions
-
Compare and contrast models with and without sidechains
-
Record the side chains from the N-terminus to the C-terminus
-
Identify the chemical properties of each side chain
-
Consider how the side chain properties impact protein structure and function
-
Analyze how regions rich in hydrophobic or polar sidechains orient within actual proteins to support structural stability
Both sets of models were lifted from the following proteins, which are shown in the slide deck:
-
The alpha-helix model represents amino acids 99-111 of chain B of the hemoglobin structure file 1a3n.pdb
-
The beta-sheet model represents amino acids 13-33 and 119-127 of chain A of the GFP structure file 1emb.pdb






 Account
Account Shopping Cart
Shopping Cart